Phosphorus is a chemical element that can be found in two different forms naturally, either as red phosphorus or white phosphorus. Due to its highly reactive nature, phosphorus cannot be found in its free elemental form on the planet Earth.
Phosphorus is the second member of the pnictogen group (the elements that correspond to group 15 of the modern periodic table). The atomic number of phosphorus is 15. Therefore, the electronic configuration of phosphorus can be written as [Ne]3s23p3. Phosphorus can be classified as a reactive nonmetal. Under standard conditions for temperature and pressure, phosphorus generally exists in the solid phase. Some important chemical compounds that contain phosphorus are listed in this article.
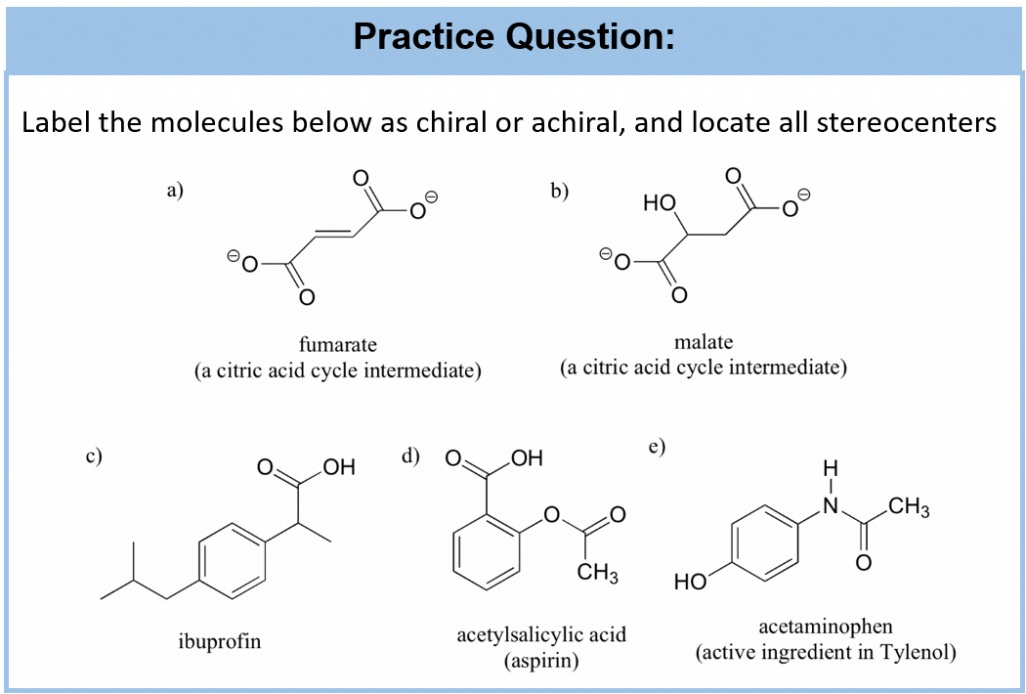
Phosphorus Trichloride
Phosphorus trichloride is a halide of phosphorus which is denoted by the chemical formula PCl3. Under standard conditions, this compound exists as a highly volatile and toxic liquid that tends to undergo a violent chemical reaction when exposed to water, forming hydrochloric acid in the process. The molecular structure of phosphorus trichloride is trigonal pyramidal. The molar mass of phosphorus trichloride is roughly equal to 137.33 grams per mole. This compound is often associated with a very unpleasant acrid odour that is quite similar to the odour of hydrochloric acid. The density of phosphorus trichloride is approximately equal to 1.574 grams per cubic centimetre. It is important to note that phosphorus trichloride can act as a Lewis base since it possesses a lone pair of electrons.
Adenosine Triphosphate (ATP)
Adenosine triphosphate is an organic compound that is broken down to provide energy for several life-sustaining processes in cells. ATP is found in all known life forms that exist on the planet Earth. The chemical formula of adenosine triphosphate can be written as C10H16N5O13P3. The molar mass of adenosine triphosphate is roughly equal to 507.18 grams per mole.
Phosphoric Acid
Phosphoric acid is a weak acid of phosphorus which is denoted by the chemical formula H3PO4. This compound is sometimes referred to as phosphoric (V) acid and as orthophosphoric acid. The molar mass of phosphoric acid is equal to 97.994 grams per mole. Under standard conditions for temperature and pressure, phosphoric acid is known to exist as a colourless syrup that does not have any characteristic odour. The density of this compound corresponds to 1.6845 grams per cubic centimetres in its syrup form. However, solid phosphoric acid is known to have a density of 1.834 grams per cubic centimetre. The boiling point of phosphoric acid is roughly equal to 212 degrees Celsius. This compound is quite soluble in water, with a solubility of roughly 548 grams per 100 millilitres at a temperature of 20 degrees Celsius. The primary application of phosphoric acid is in the production of fertilizers for crops (since phosphorus is an essential nutrient for plants to grow). Another vital application of this compound is in the production of a wide spectrum of soaps and detergents.
To learn more about the properties of phosphorus atoms and other important topics in chemistry such as atom definition, download BYJU’S – The Learning App.